Explain the Difference Between Filtration and Decantation
Separation Technique There are numerous methods of separating two components and the technique depends upon the properties of the. Filtration can be described as an act or process of filtering or cleaning something.

Difference Between Filtration And Decantation Explained
100 2 ratings Filtration is the direct separation of a solid precipitate and the solvent in which it is contained by th.

. View the full answer. Filtration on the other hand is a process of separating solids from liquids by passing it through a medium only a liquid can pass. Decantation and filtration differ in the manner through which the methods separate substances in a mixture.
The key difference between decantation and filtration is that decantation separates two components in a mixture by pouring off one component whereas filtration separates two components by filtering off one component. Learn vocabulary terms and more with flashcards games and other study tools. In decantation when we pour out supernatant liquid there are chances of their getting mixed up with solute particles.
The major difference between decantation and filtration is in the process by which it is achieved. Besides the filtrate obtained is generally pure solvent. Why might one want to use filtration in this experiment rather than decantation.
Like mud settles from muddy water. Decantation is the method of cleaning in which we dont use a medium which has. Why might one want to use filtration in this experiment rather than decantation.
The mixture is just poured in a vessel through a filter paper. The key difference between decantation and filtration is that decantation separates two components in a mixture by pouring off one component whereas filtration separates two components by filtering off one component. The major difference between decantation and filtration is in the process by which it is achieved.
Difference Between Decantation and Filtration. Decantation is the process of pouring out the liquid. It is defined as the separation process in which two immiscible liquids are separated.
This is done by pouring out the clear upper layer of liquid. In filtration you are just dumpinf a mixed amount of substances through some pours and in ten seconds you are done. Sedimentation - Sedimentation is the process in which heavier particles of an insoluble solid settle down in a liquid.
Correct answer to the question Explain the difference between filtration and decantation see the general laboratory equipment and procedures section of this manual. In decantation the mixture is left undisturbed for some time and then the liquid is poured in another vessel without disturbing the sediments. Experts are tested by Chegg as specialists in their subject area.
Like draining oil from a mixture of oil and water after allowing the two to separate and form distinct layers. Explain the difference between filtration and decantation see the General laboratory Equipment and Procedures section of this manual. It cannot be used to separate two liquids.
It is a process by which one can separate mixtures of substances. Once the substances have separated the lighter substance is poured out leaving the heavier. We can remove sand from water through the process of sedimentation.
Answered on 1st Feb 2021. Filtration is a better technique than sedimentation and decantation because it can separate very Fine insoluble particles as compared to the latter. We review their content and use your feedback to keep the quality high.
Decantation is pouring out of upper clear layer of liquid into another container to separate two immiscible liquids. This process can also be used to separate two liquids that do not mix eg oil and water. The key difference between sedimentation and decantation is that the sedimentation allows the separation of two substances via settling of one substance whereas the decantation allows the separation of two substances via pouring off one substance.
Filtration on the other hand is a process of separating solids from liquids by passing it through a medium only a liquid can pass. FILTRATION IS faster. Whereas in case of filtration the mixture is not left undisturbed for sediments to settle down.
Filtration is the process of separating insoluble impurities from a solution. Decantation is a process for the separation of mixtures of immiscible liquids and solid such as suspension. Start studying Unit 42 Decantation and Filtration.
Both sedimentation and decantation are important separation methods in analytical chemistry. Both decantation and filtration separate two components in a liquid-solid mixture or a mixture of two immiscible liquids under. By means of decantation you have to slowly.
Decantation is pouring away a liquid from solid impurities which have. Why might one want to use filtration in this experiment rather than decantation. Filtration is the direct separation of the entire solution through a filter where the solid is trapped by the filter allowing the liquid to pass through.
While both filtration and decantation can be used to separate impurities from liquids there are differences between them. Explain the difference between filtration and decantation see the General laboratory Equipment and Procedures section of this manual. Explain the difference between filtration and decantation.
Separation of a mixture of oil from water is an example of decantation. Decantation is a process for the separation of mixtures of immiscible liquids and solid such as suspension. In decantation the separation of two substances either of a solid and liquid or two immiscible liquids occurs by allowing the mixture to settle and separate.
Difference Between Filtration and Decantation Explained Filtration.

Difference Between Decantation And Filtration Compare The Difference Between Similar Terms

Difference Between Decantation And Filtration Compare The Difference Between Similar Terms
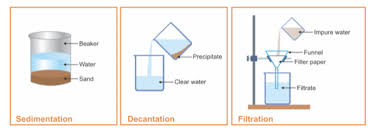
What Is Sedimentation Decantation And Filtration Home Work Help Learn Cbse Forum

No comments for "Explain the Difference Between Filtration and Decantation"
Post a Comment